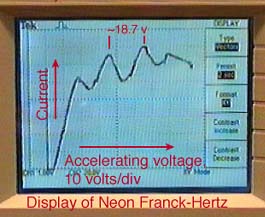
The Franck-Hertz Experiment*
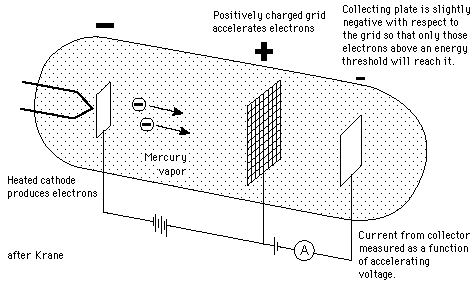
In 1914, James Franck and Gustav Hertz performed an experiment
which demonstrated the existence of excited states in mercury atoms,
helping to confirm the quantum theory which predicted that electrons
occupied only discrete, quantized energy states. Electrons were
accelerated by a voltage toward a positively charged grid in a glass
envelope filled with mercury vapor. Past the grid was a collection
plate held at a small negative voltage with respect to the grid. The
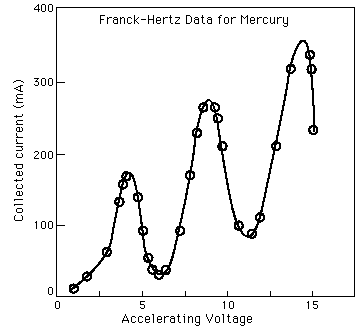
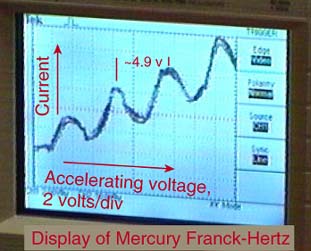
values of accelerating voltage where the current dropped gave a measure
of the energy necessary to force an electron to an excited state. 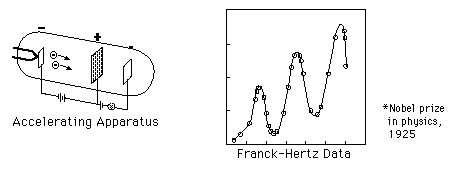
*Nobel Prize in physics, 1925
| Further discussion | Sketch of apparatus | Data for mercury | Data for neon |